"All models are wrong, but some are useful." -George Box
"Well... You see... When its a particle it spins. When its a wave its still doing that. How does a waveform spin you ask? Listen. Shut the fuck up. The math is really weird and some of this stuff just happens and you can't visualize it in your head. We didn't believe it at first either but after 50 years of experiments we have to just accept that reality is consistent with the math even if we don't fully conceptualize what that means even"
We are all just folds in this wonderfully weird thing we call spacetime!
Nice reference to PBS Space Time. The YouTube channel where I just get bullied with science, and for some weird twisted reason I like it.
pbs space time is awesome, and this description is even more so.
Bullied with science sounds like a fun band name
The prions of spacetime.
Out here folding along.
- When it's* a particle
- When it's* a wave
- it's* still doing that
You seem to be up to date with this stuff; did we find out whether there's more than one yet..?
Personally don't like the idea of everyone reusing the same electon for everything... seems quite unhygienic. I'd rather we had at least one per person, maybe share it with people we trust, if we must...
We have to recycle nowadays. Besides we can't have people throwing away perfectly good electrons. They could end up anywhere.
I swear quantum physics is magic and made up!
Magnets, how do they work?
Also please don't look at it
I mean, you can but it won’t be there.
Actually, it can be there, but then you won't know how fast it's moving.
think of it as a camera.
if you set it up with a high speed to take a picure of a bouncing ping pong ball you will know its precise location at the moment of the shot.
if you set it up with a low speed you will see a blur of the path it took, but not a precise location.
That's not a good analogy because typically cameras don't change the things they're observing. But, a camera with a flash...
Imagine a guy driving down a dark road at night. Take a picture of him without a flash and you'll get a blurry picture.
Take a picture of him with a powerful flash and you'll get an idea of exactly where he was when the picture was taken, but the powerful flash will affect his driving and he'll veer off the road.
You can't measure something without interacting with it. This is true even in the non-quantum world, but often the interactions are small enough to ignore. Like, if you stick a meat thermometer into a leg of lamb, you'll measure its temperature. But, the relatively cool thermometer is going to slightly reduce the temperature of the lamb.
At a quantum level, you can no longer ignore the effect that measuring has on observing. The twin-slit experiment is the ultimate proof of this weirdness.
You see, wire telegraph is a kind of a very, very long cat. You pull his tail in New York and his head is meowing in Los Angeles. Do you understand this? And radio operates exactly the same way: you send signals here, they receive them there. The only difference is that there is no cat.
Google "Electron Orbitals". All the spaces there are all the ~~possible~~ highest likely locations for the electrons. Good Introduction to some Quantum Mechanics 👍
No! I will not relive the horrors of that chemistry class again... you can't make me. I am happily an aerospace engineer now where I don't need this chemistry nonsense, or quantum mechanics.
Ah let's see, of the top of my head...
~~1s² 2s² 2d⁶ 3s² 2p¹⁰ ...~~
Edited (iirc now, the d block is in the middle with the transition metals, p block with metallics, Halogens, Noble Gases...):
1s² 2s² 2p⁶ 3s² 2d¹⁰ ...
![]()
LMAO
This I was fine with. But that fake make believe redox math? Like are all chemist bad at actual math, so they just came up with their own fake version?
Except they only look like that if there is an external reference system imposing some structure on the atom! Otherwise all orbitals are basically spherical because they can all just be in a superposition of all possible orbitals and we couldn't tell a difference...
And then suddenly you have two atoms meeting and need to explain why 1+1=0 for their molecular orbits -.-
I don't think so. Orbitals give you the spaces of highest probability! Electrons could be outside as well. And since it is based on probability it is definitely a useful model.
Electronic orbitals are regions within the atom in which electrons have the highest probability of being found.
My advanced E&M professor said "Imagine a sphere of radius zero. Trust me, it works."
"...Imagine a sphere of radius zero."
and a spherical cow. imagining lots of spherical cows helps quite a bit.
Radiating milk equally in all directions, of course.
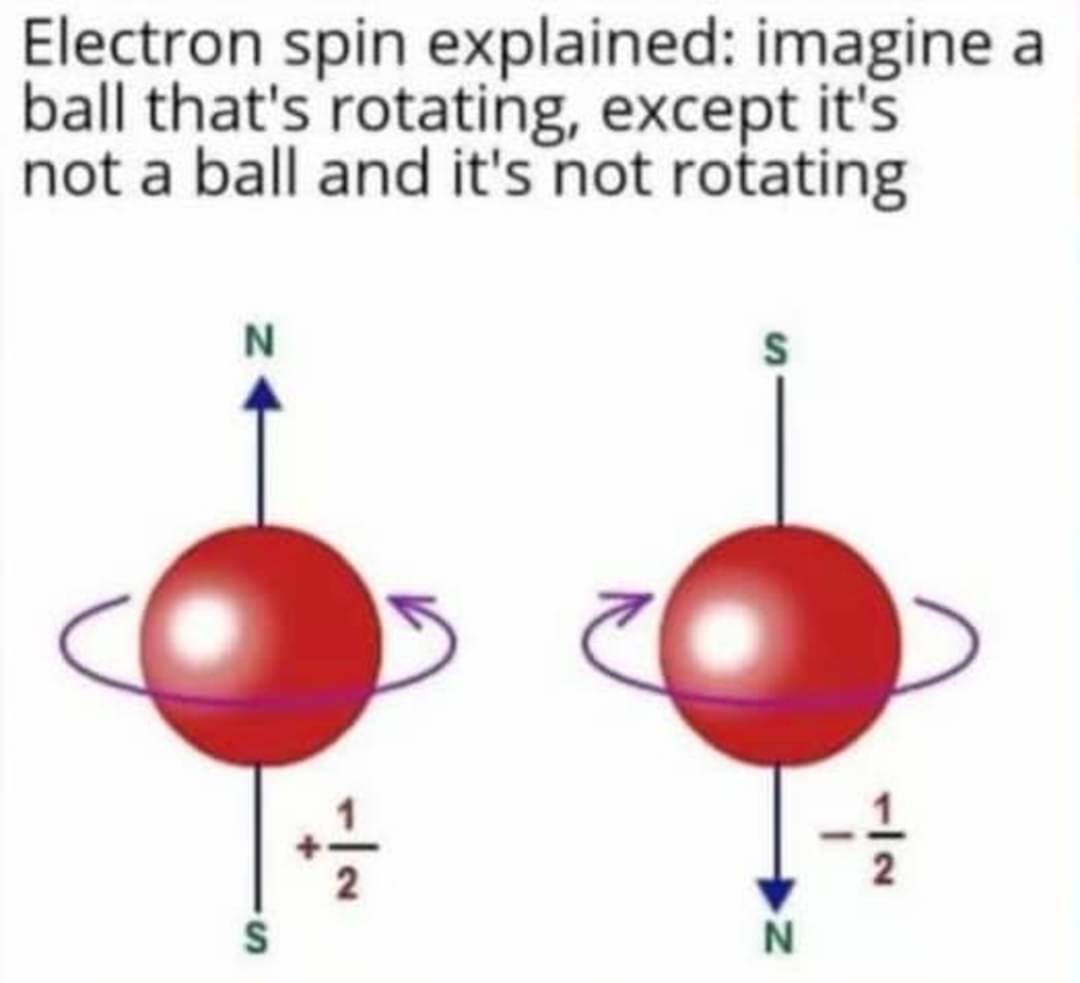
+1/2 h and -1/2 h
Fucking hate the people that insist on using only half of the number as if it was a real value. At least say you are working with natural unities or something.
" - How far is your house? - Oh, it's just 5!"
Except in this context the question is "how many blocks away is your house?" Where "5" is a completely valid response
It's h-bar, not h. And it really does make sense if you look deeper I to the math.
Using "+1/2" and "-1/2" as vector labels is fine. Using it on the context of "the spin can have those 2 values here" for laypeople without further explanation is just making the subject less accessible.
Also, yeah, I was too lazy to search for the unicode ħ.
It's a point but it doesn't actually exist at any point. It exists in a cloud where it could exist anywhere in there.
You can observe it but doing so changes its behavior. Why? Well... Um... Maybe it's just the simulation breaking down?
It's because to observe something you have to interact with it. Dealing with particles is like playing pool in the dark and the only way you can tell where the balls are is by rolling other balls into them and listening for the sound it makes. Thing is, you now only know where the ball was, not what happened next.
In the quantum world, even a single photon can influence what another particle is doing. This is fundamentally why observation changes things.

holy shit the pool explanation is so good, I'm gonna recycle it for sure
Good metaphor
I think a lot of the confusion people have is around the word “observation” which in everyday language implies the presence of an intelligent observer. It seems totally nonsensical that the outcome of a physics experiment should depend on whether the physicist is in the lab or out for a coffee! That’s because it is!
I have this beef with a lot of words used in physics. Taking an everyday word and reusing it as a technical term whose meaning may be subtly and/or profoundly different from the original. It’s a source of constant confusion.
The closest representation is that cliche television shot where someone's thinking really hard and equations fly around their head.
Science Memes
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]