this post was submitted on 25 Oct 2024
705 points (96.2% liked)
Science Memes
11021 readers
3452 users here now
Welcome to c/science_memes @ Mander.xyz!
A place for majestic STEMLORD peacocking, as well as memes about the realities of working in a lab.

Rules
- Don't throw mud. Behave like an intellectual and remember the human.
- Keep it rooted (on topic).
- No spam.
- Infographics welcome, get schooled.
This is a science community. We use the Dawkins definition of meme.
Research Committee
Other Mander Communities
Science and Research
Biology and Life Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- !reptiles and [email protected]
Physical Sciences
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
- [email protected]
Humanities and Social Sciences
Practical and Applied Sciences
- !exercise-and [email protected]
- [email protected]
- !self [email protected]
- [email protected]
- [email protected]
- [email protected]
Memes
Miscellaneous
founded 2 years ago
MODERATORS
you are viewing a single comment's thread
view the rest of the comments
view the rest of the comments
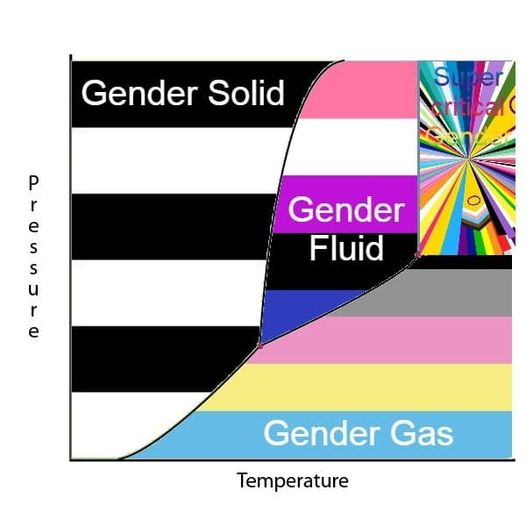
Hey guys. In high school I graduated with fantastic grades in physics. I have a BSc in Chemical Engineering. Can somebody explain supercritical fluids to me please
A fluid gets too hot and compressed that it forgets it's supposed to be either a liquid or a gas, so it decides to be both.
what does that mean though. Are they still compressible?
A supercritical fluid is a fluid that shows properties of both a gas and a liquid. It happens because the pressure and temperature of that fluid happen to be in the region where the line between liquid and gas becomes blurred, and you can't tell where one state ends and the other begins.
As far as I remember, a supercritical fluid can still be compressed to some extent, though being supercritical already puts it somewhat near it's physical limit.